TrialSite Revisits Evidence for Nasopharyngeal Sanitization Research Targeting COVID-19
A co-founder of the Front Line COVID-19 Critical Care Alliance (FLCCC), recently in a communication to TrialSite Dr. Marik remarked that the efficacy of the compound (NONs) remains uncertain due to limited clinical data. Moreover, Marik reminds us that the product isn’t available in the United States.
The FLCCC co-founder reminded TrialSite about his organization’s point of view on existing, real-world data. For example, Marik’s group has been recommending nasopharyngeal sanitization for months now off-label via physician prescription and patient consent.
Marik, heavy credentialled yet controversial due to his strong stance on the mission-critically urgent need for the use of repurposed drugs early on in the life cycle of SARS-CoV-2 infection, he recently wrote to some GOP Congressmen to introduce his perspective on oropharyngeal mouthwashes and iodine nasal sprays.
Noting that they are “highly virucidal against SARS-CoV-2 and have shown to improve clinical outcomes” the critical care physician shared the results of one randomized controlled trial—a recent Bangladesh investigation shared below.
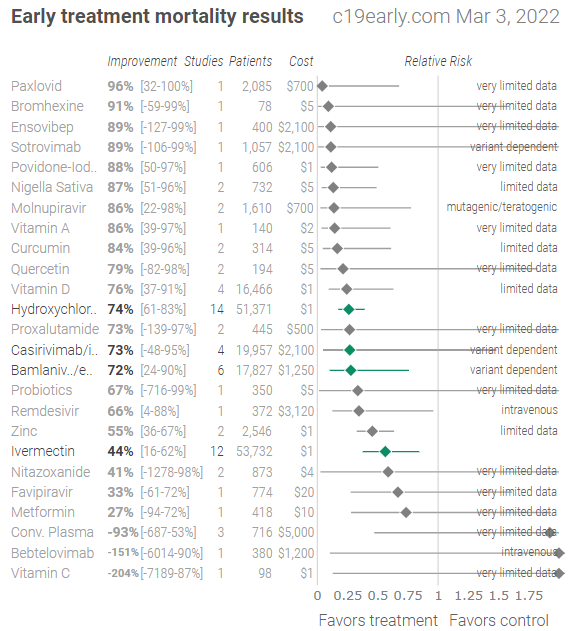
Back in late September of 2021, TrialSite featured research involving povidone-iodine against COVID-19. This media platform noted that at least some recent evidence suggests that Povidone-Iodine (PVP-I), one of the active components in mouth rinses or gargles used by dentists, successfully decreases viral levels of COVID-19 in salivary samples of patients with COVID-19.
PVP-I is the polymer polyvinylpyrrolidone complexed with iodine. As an antiviral agent, it targets the lipid envelope of several viruses, ranging from Influenza to Ebola. When the protective envelope has been compromised, viral proteins and nucleic acids can then be destroyed by lysis or oxidation.
COVID-19 viral particles recognize and attach to various receptor proteins on the human cellular membrane. One of its main targets, angiotensin-converting enzyme 2 (ACE-2) receptor, is found predominantly in the oral tissues of the body, especially on the tongue, making the oral cavity a very high-risk site for infection. The COVID-19 spike protein binds and infects human cells through this receptor. By disrupting the process, viral load and infectivity levels can be reduced in the oral cavity.
In that TrialSite piece after a review of PVP-1 as a possible early intervention against COVID-19, other research was reviewed.
Early on in the pandemic, at least some U.S.-based scientists have called for the potential prophylaxis benefits of such an approach.
One French study showcased the potential of this investigational approach alongside some risk of adverse impacts. The authors recommended a larger clinical trial to confirm the benefits while emphasizing the use of lower PI concentrations to minimize adverse effects.
What about the Bangladesh Study?
Marik himself shared with the GOP Congressmen in his email the results of a Bangladeshi study. Published in Bioresearch Communications, investigators from Dhaka-based academic medical centers shared the results of their randomized controlled trial noting out of 1113 patients, 606 were enrolled and randomized in two groups post informed consent.
The physician-scientists reported that based on the outcomes of this peer-reviewed, published study result administration of 1% PVP-I as mouthwash/gargle, nasal, or eye drop is simple, rapid, and cost-effective in reduction of mortality and morbidity by COVID-19.
One Physician-Scientist Point of View
TrialSite interacted with Dr. Marik via email to elicit his latest thinking on what is considered an investigational approach—again it’s not indicated for COVID-19 via the U.S. Food and Drug Administration (FDA).
Dr. Marik shared with TrialSite “Povidone-iodine is highly virucidal against SARS-CoV-2. It kills the virus within seconds. This simple cheap nasal spray plays an important adjunctive role in post-exposure prophylaxis and acute symptomatic COVID-19 infection.”
Presently 24 studies are disclosed in the U.S. clinical trials registry involving PI and COVID-19.
Dr. Marik makes his organization’s protocol available to other physicians at the Front Line COVID-19 Critical Care Alliance website. This includes the “I-MASK+ Prevention & Early Outpatient Treatment Protocol for COVID-19.” These protocols are not recognized by the regulators and require licensed physicians with consenting patients. Moreover, during COVID a top-down national government intervention has led to charges involving physician harassment and worse. During COVID real-world data involving rebranded drugs has been shunned by apex research institutions and regulators not to mention powerful physician societies.
- Povidone 10% Iodine on Amazon (Betadine)
- Povidone 10% Iodine on Amazon (Amazon Brand)






.png)

Comments
Post a Comment