Spike Protein Syndrome Treatment: 7 Case Reports (2025)
More than four years after COVID-19 first appeared, many people still suffer from long-lasting symptoms after getting infected. This ongoing condition was often called 'Long COVID'. It means symptoms stick around for three months or more after the infection or after exposure to certain COVID-19 vaccines that use mRNA technology.
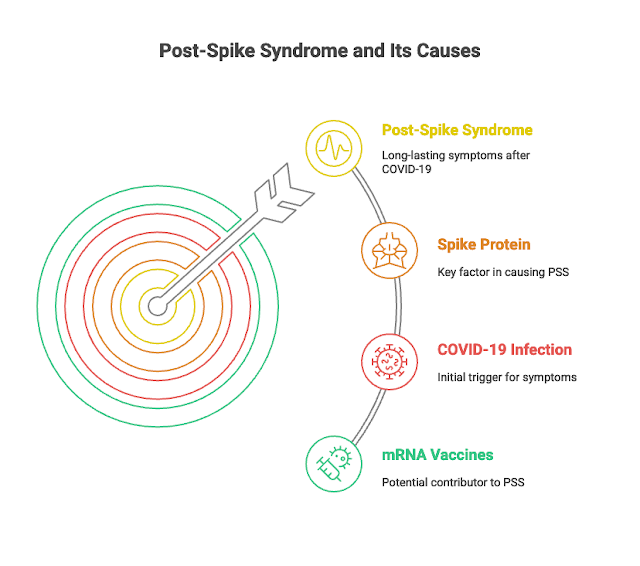
In late 2023, some researchers introduced a new name, "SPIKEOPATHY," to explain that a protein called the Spike protein—found in both the virus and mRNA vaccines—plays a big role in causing these long-term problems. Because of this, the term Post-Spike Syndrome (PSS) is suggested as a clearer name for this condition.
Sometimes, the Spike protein appears on our own cells, which can confuse the immune system and cause it to attack the body by mistake. This can make inflammation worse.
Our goal is to help both patients and doctors recognize Post-Spike Syndrome (PSS), suggest a possible treatment approach, and encourage more research to better understand and manage this often long-lasting and challenging condition.
Case No. 1
A 57-year-old female presented in November 2024 with worsening skin lesions in the elbow region since August 2021. The patient had experienced similar but milder lesions since 2018. Additionally, she reported intense fatigue, alopecia, memory decline, and tingling in the extremities, which are classic symptoms of Post-Spike Syndrome (PSS). A biopsy from April 2024 confirmed interstitial granulomatous dermatitis (IGD).
Despite consulting several dermatologists and undergoing extensive workup, no underlying pathology linked to IGD was identified. A trial of corticosteroids was attempted but produced no improvement.
Upon reviewing her history, we identified a correlation between her PSS symptoms and IGD flare-ups following doses of Pfizer mRNA-based immunotherapy (she received two doses from Pfizer, one in August and another in December 2021). We (Zeballos et al 2025) initiated antibiotics for five days using criteria according to the patient's individual characteristics. In this case, we used:
We (Zeballos et al 2025) initiated our therapeutic strategy (below) in addition to Her existing medications (levothyroxine, losartan 50 mg twice daily, amlodipine 5 mg, rosuvastatin 20 mg, paroxetine 20 mg, and alprazolam 0.5 mg). After two months of treatment, most PSS symptoms regressed, with only mild residual tingling in her right hand. However, in March 2024, she experienced a relapse of PSS. Upon restarting treatment, symptoms improved again, except for the persistent tingling in her left hand and around her mouth, which remained mild but unresolved. As of November 24, 2024, the patient is stable.
After contracting COVID-19 in September 2021, she developed fatigue, memory loss, joint pain, intestinal problems, and tingling in the lips, consistent with PSS. Her seizures were myoclonic and incapacitating, prompting her to consult multiple specialists. She then received hospital medical care aimed at controlling the seizures and adjusting her medication, but they were all without success.
This patient has not been vaccinated for COVID-19. She saw six neurologists and three epilepsy specialists, all of whom failed to control her seizures. At one point, she was taking eleven anticonvulsants daily,which either failed to prevent seizures or caused excessive sedation, which led her to stop taking the anticonvulsants. Eventually, she opted to manage the seizures by sitting or lying down as soon as she noticed the visual aura. She ended up having four seizures per week. In August 2022, her condition worsened, leading to hospitalization for severe myoclonic seizures. A third COVID-19 infection in May 2024 triggered another increase in seizure frequency and PSS symptoms, including fatigue, memory loss, joint pain, and intestinal issues.
Weight was measured at 172 pounds, with a body mass index of 24.4 kg/m². Auscultation revealed no pulmonary crackling or rales, and there were no heart murmurs. SARS-CoV-2 S protein IgG quantification was 7058 AU/ml. Based on the presentation, a diagnosis of COVID-19 vaccine-induced myopericarditis was made. A "Spike detoxification protocol" was initiated, consisting of bromelain (500 mg once daily), nattokinase (2,000 FU twice daily), and curcumin (500 mg twice daily). The suspected inflammatory etiology, colchicine (0.6 mg/day) was also prescribed. The patient’s response to these treatments was variable, with periods of symptom improvement followed by relapses through the remainder of 2023 and into early 2024. In addition to pharmacological interventions, aerobic exercise (to the extent tolerable) and intermittent fasting were incorporated to maintain cardiac capacity and reduce systemic inflammation.
In late 2023, some researchers introduced a new name, "SPIKEOPATHY," to explain that a protein called the Spike protein—found in both the virus and mRNA vaccines—plays a big role in causing these long-term problems. Because of this, the term Post-Spike Syndrome (PSS) is suggested as a clearer name for this condition.
What is Post-Spike Syndrome?
PSS can affect many parts of the body and cause serious, long-lasting health problems. Scientists are learning more about how the Spike protein causes these issues. The tiny particles from mRNA vaccines can travel through the body, not just stay where the shot was given. Both these particles and the Spike protein can trigger inflammation (the body's reaction to injury or infection) in different organs.Sometimes, the Spike protein appears on our own cells, which can confuse the immune system and cause it to attack the body by mistake. This can make inflammation worse.
Symptoms of PSS
Because the Spike protein spreads through the bloodstream, it can affect many systems in the body, including:- Nervous system: causing brain fog, memory problems, headaches, tingling in hands or feet
- Heart: irregular heartbeats
- Immune system: flare-ups of existing diseases like psoriasis or autoimmune disorders
- Digestive system: nausea, diarrhea, loss of appetite
- Skin: rashes, hair loss
- Eyes: vision problems
- Other symptoms: extreme tiredness, joint pain, sleep problems
Post-Spike Syndrome or Long Covid Case Series Compilation
The following case reports were collected from different peer-reviewed journals, websites and social media platforms, offering real-life stories and experiences shared by the public, doctors and researchers.
Our goal is to help both patients and doctors recognize Post-Spike Syndrome (PSS), suggest a possible treatment approach, and encourage more research to better understand and manage this often long-lasting and challenging condition.
Case No. 1
A 57-year-old female presented in November 2024 with worsening skin lesions in the elbow region since August 2021. The patient had experienced similar but milder lesions since 2018. Additionally, she reported intense fatigue, alopecia, memory decline, and tingling in the extremities, which are classic symptoms of Post-Spike Syndrome (PSS). A biopsy from April 2024 confirmed interstitial granulomatous dermatitis (IGD).
Despite consulting several dermatologists and undergoing extensive workup, no underlying pathology linked to IGD was identified. A trial of corticosteroids was attempted but produced no improvement.
Upon reviewing her history, we identified a correlation between her PSS symptoms and IGD flare-ups following doses of Pfizer mRNA-based immunotherapy (she received two doses from Pfizer, one in August and another in December 2021). We (Zeballos et al 2025) initiated antibiotics for five days using criteria according to the patient's individual characteristics. In this case, we used:
- Ciprofloxacin 500 mg, administered once a day for 5 days. (Fluoroquinolone Alert)
- Nattokinase 100 mg twice a day for at least 90 days
- Ivermectin 6 mg/30 kg administered 3 times a week for at least 8 weeks
- Probiotics (Lactobacillus acidophilus NCFM®, Lactobacillus paracasei Lpc−37™, Bifidobacterium lactis BI−04™, Bifidobacterium lactis BI−07™ and Bifidobacterium bifidum Bb−02™) 335 mg twice a day for 90 days.
Case 2
A 44-year-old female consulted in June 2023, reporting intense fatigue, alopecia, memory decline, brain fog, depression, and vertigo since her first COVID-19 infection in October 2022. These symptoms worsened after a second COVID-19 infection in February 2023. Around the same period, she developed severe and disabling trigeminal neuralgia, which had never occurred before. Patient reports receiving one dose of Pfizer vaccine in July 2021.
We (Zeballos et al 2025) initiated our therapeutic strategy (below), in addition to pregabalin and opioids for severe neuralgia. Considering a potential subclinical herpesvirus reactivation, we also prescribed valacyclovir alongside analgesics. After 60 days of treatment, the PSS symptoms completely resolved. Notably, trigeminal neuralgia entered remission within 30 days and has remained so as of may 28, 2025.
Antibiotics for five days using criteria according to the individual characteristics of the patient. In this case, we used:- Ciprofloxacin 500 mg, administered once a day. (Fluoroquinolone Alert)
- Nattokinase 100 mg twice daily for at least 90 days
- Ivermectin 6 mg/30 kg administered 3 times weekly for at least 8 weeks
- Probiotics (Lactobacillus acidophilus NCFM®, Lactobacillus paracasei Lpc−37™, Bifidobacterium lactis BI−04™, Bifidobacterium lactis BI−07™ and Bifidobacterium bifidum Bb−02™) 335 mg twice daily for 90 days.
Case 3
A 52-year-old female consulted in August 2024, having contracted COVID-19 in February 2024. One month post-infection, she developed extreme fatigue, shortness of breath, intestinal issues, and alopecia, consistent with PSS. Additionally, she experienced muscle pain in the shoulders and pelvic girdle, along with cervical stiffness. This patient has not been vaccinated for COVID- 19. A rheumatologist diagnosed polymyalgia rheumatica in June 2024, after ruling out differential diagnoses. Laboratory tests showed C-reactive protein (CRP) at 10.4 mg/L and erythrocyte sedimentation rate (ESR) at 40 mm/hr. She was prescribed prednisolone (10 mg/day) in July 2024, which helped with pain but failed to resolve other symptoms, including brain fog, fatigue, and intestinal issues. We (Zeballos et al 2025) initiated our therapeutic strategy (below) three weeks after corticosteroid therapy began. After 60 days of treatment, the PSS symptoms resolved, and inflammatory markers improved significantly.This led her rheumatologist to reduce prednisolone from 10 mg to 2 mg/day.
As of December 8, 2024, the patient remains stable and nearly pain-free. Her rheumatologist was surprised by the rapid improvement, as standard treatment for polymyalgia rheumatica typically takes about a year to yield similar results.
Antibiotics for five days using criteria according to the patient's individual characteristics. In this case, we used: - Ciprofloxacin 500 mg, administered twice a day. (Fluoroquinolone Alert)
- Nattokinase 100 mg twice a day for at least 90 days
- Ivermectin 6 mg/30 kg administered 3 times a week for at least 8 weeks
- Probiotics (Lactobacillus acidophilus NCFM®, Lactobacillus paracasei Lpc−37™, Bifidobacterium lactis BI−04™, Bifidobacterium lactis BI−07™ and Bifidobacterium bifidum Bb−02™) 335 mg twice a day for 90 days.
Case 4
A 70-year-old female consulted in July 2023. She experienced a severe Reaction with tremors and hypothermia after her first dose of AstraZeneca in April 2021. Thirty days later, she developed tingling in one finger of her left hand. The patient received another dose of AstraZeneca in April 2021 and a Pfizer in January 2022. After her second COVID-19 infection in September 2023, she developed widespread tingling (especially in her hands, feet, legs, and around her mouth), burning during bowel movements, brain fog, dizziness, and intense fatigue, consistent with PSS.We (Zeballos et al 2025) initiated our therapeutic strategy (below) in addition to Her existing medications (levothyroxine, losartan 50 mg twice daily, amlodipine 5 mg, rosuvastatin 20 mg, paroxetine 20 mg, and alprazolam 0.5 mg). After two months of treatment, most PSS symptoms regressed, with only mild residual tingling in her right hand. However, in March 2024, she experienced a relapse of PSS. Upon restarting treatment, symptoms improved again, except for the persistent tingling in her left hand and around her mouth, which remained mild but unresolved. As of November 24, 2024, the patient is stable.
In this case, antibiotic therapy was not used, as the patient had recently taken this type of medication, 14 days before our consultation. She used Amoxicillin + Potassium Clavulanate 875 mg, which she did not tolerate. Therefore, she switched to moxifloxacin hydrochloride 400 mg for 14 days, starting on 10/19/2024. Other treatments:
- Nattokinase 100 mg twice a day for at least 90 days
- Ivermectin 6 mg/30 kg administered 3 times a week for at least 8 weeks
- Probiotics (Lactobacillus acidophilus NCFM®, Lactobacillus paracasei Lpc−37™, Bifidobacterium lactis BI−04™, Bifidobacterium lactis BI−07™, and Bifidobacterium bifidum Bb−02™) 335 mg twice a day for 90 days.
Case 5
A 50-year-old female, consulted in September 2024 with a history of Encephalitis in March 2021, for which COVID-19 was ruled out despite extensive testing. Following encephalitis, she developed seizures responsive only to valproic acid, which caused alopecia and sedation.After contracting COVID-19 in September 2021, she developed fatigue, memory loss, joint pain, intestinal problems, and tingling in the lips, consistent with PSS. Her seizures were myoclonic and incapacitating, prompting her to consult multiple specialists. She then received hospital medical care aimed at controlling the seizures and adjusting her medication, but they were all without success.
This patient has not been vaccinated for COVID-19. She saw six neurologists and three epilepsy specialists, all of whom failed to control her seizures. At one point, she was taking eleven anticonvulsants daily,which either failed to prevent seizures or caused excessive sedation, which led her to stop taking the anticonvulsants. Eventually, she opted to manage the seizures by sitting or lying down as soon as she noticed the visual aura. She ended up having four seizures per week. In August 2022, her condition worsened, leading to hospitalization for severe myoclonic seizures. A third COVID-19 infection in May 2024 triggered another increase in seizure frequency and PSS symptoms, including fatigue, memory loss, joint pain, and intestinal issues.
After one week of treatment, her seizures significantly improved, and after three weeks, all PSS symptoms resolved. The patient experienced complete remission of seizures and PSS symptoms, remaining stable as of November 24, 2024.
- Ciprofloxacin 500 mg, administered twice a day. (Fluoroquinolone Alert)
- Nattokinase 100 mg twice daily for at least 90 days
- Ivermectin 6 mg/30 kg administered 3 times weekly for at least 8 weeks
- Probiotics (Lactobacillus acidophilus NCFM®, Lactobacillus paracasei Lpc−37™, Bifidobacterium lactis BI−04™, Bifidobacterium lactis BI−07™ and Bifidobacterium bifidum Bb−02™) 335 mg twice daily for 90 days.
Case No. 6
- A 21-year-old male diagnosed with autism spectrum disorder (ASD) and attention deficit hyperactivity disorder (ADHD) received one dose of the Moderna mRNA-1273 COVID-19 vaccine (100 mcg - batch: 061E21A) on November 18th, 2021.
- Approximately two weeks later, the patient ingested a supratherapeutic dose of atomoxetine (ATX, 200 mg) and dexmethylphenidate (d-MPH, 150 mg, Focalin®), having previously taken similar doses prior to vaccination, which resulted in expected sympathomimetic effects including xerostomia, melanoglossia, and anxiety, without any cardiac symptoms.
- The patient subsequently developed hyperhidrosis, orthostatic intolerance, subjective palpitations, and pitting lower extremity edema, which spontaneously resolved within 24 hours, and the patient returned home without seeking medical attention.
- The patient experienced recurring cardiovascular symptoms, particularly after the ingestion of d-MPH/ATX, prompting discontinuation of psychostimulants and atomoxetine.
- Throughout the following months, the patient’s condition deteriorated, with frequent episodes of angina pectoris, dyspnea, palpitations, edema, and fatigue without clear inciting factors.
- The patient sought emergency department care multiple times, but symptoms were consistently attributed to anxiety, likely due to his history of anxiety diagnoses.
- Nine months after mRNA injection, the patient suffered vaccine failure and contracted COVID-19, managed with nirmatrelvir/ritonavir.
- The infection was moderate, peaking with a temperature of 104°F, and was followed by several non-SARS-CoV-2 viral upper respiratory infections.
- Seven months after mRNA injection, he contracted COVID-19 again, and while the infection was mild, he soon developed symptoms of heart failure (NYHA Class III), including effort intolerance, dyspnea, orthostatic intolerance, and lower extremity edema.
- 48-hour ambulatory ECG revealed a heart rate range of 39-205 bpm, with consistent sinus rhythm.
- Cardiac Evaluation:
- 48-hour ambulatory ECG revealed a heart rate range of 39-205 bpm, with consistent sinus rhythm.
- Blood pressure was 128/90 mmHg, pulse rate was 62 bpm, and SpO2 was 100% as clinically measured.
- Echocardiography showed an ejection fraction of 60%.
- Electrocardiogram (ECG) at initial clinical encounter indicated diffuse ST elevation.
6-Month Follow-Up and Successful Treatment with Rapamycin:
Dr Robert W. Enzenauer, MD, MPH shared in September 2024:
Dr. McCullough : I am a physician who got Pfizer 2020, 2021 and 2021 to keep working at hospital in Colorado. I am happy to report: MARCH 2024 Spike ABY 1:25,000. Using the McCullough protocol now six months, my Spike antibody down to 1:740 in SEPT 2024. Thank you sir.
- After six months of treatment for myopericarditis, the patient presented with a blood pressure of 122/80 mmHg, a pulse rate of 75 bpm, and an SpO2 level of 100%.
- The ECG revealed persistent ST elevation characteristic of active, ongoing myopericarditis (Figure 4).
- Thoracic ultrasound did not reveal any pericardial effusion, and auscultation was unremarkable for abnormal heart sounds.
- The patient reported atypical chest pain localized to the seventh left intercostal space.
- A cardiac MRI with contrast was declined by the patient due to concerns about gadolinium-based contrast agents.
- SARS-CoV-2 Spike protein IgG quantification had decreased to 2650 AU/ml.
- Prednisone (20 mg/day) was initiated, and the patient experienced improvement in symptoms and resolution of the ST elevation after completing the corticosteroid course.
- Due to unfavorable cognitive side effects, the patient chose not to continue corticosteroid therapy, and alternative treatments were explored.
- Given the patient’s reluctance to continue corticosteroids and the persistence of myopericarditis symptoms, rapamycin (1 mg/day per os) was introduced as an alternative therapeutic intervention.
- The patient’s HR, SpO2, and ECG were monitored regularly during treatment.
- After approximately four weeks of rapamycin administration, ST elevation resolved, and QRS voltage returned to normal (previously showing low voltage).
- Subjectively, the patient reported durable resolution of symptoms, including chest pain, dyspnea, and effort intolerance.
- Follow-up labs, including a complete blood count (CBC) and comprehensive metabolic panel (CMP), were performed three months after rapamycin initiation and were unremarkable.
- A repeat ECG at seven months into rapamycin treatment showed persistent resolution of the ST elevation.
- The patient’s condition had stabilized, and no significant side effects from rapamycin, such as depression of white blood cell counts or inhibition of wound healing, were observed.
Case No. 7
Dr Robert W. Enzenauer, MD, MPH shared in September 2024:
Dr. McCullough : I am a physician who got Pfizer 2020, 2021 and 2021 to keep working at hospital in Colorado. I am happy to report: MARCH 2024 Spike ABY 1:25,000. Using the McCullough protocol now six months, my Spike antibody down to 1:740 in SEPT 2024. Thank you sir.
Sources and References:
.png)
.png)






.png)

Comments
Post a Comment