Blood Clotting May Be Causing Long COVID and Vaccine Injury: How to Get Better
“I had barely sat down in the monitoring station, and my bottom barely hit the chair when I was already asking for a nurse,” Dobbs told The Epoch Times.
“I was having immediate heart palpitations, racing heart, increased pulse respirations. And by the time the nurse could get the machine over to hook me up for blood pressure, pulse, and oxygen and all that, my blood pressure was so high … I don’t know how I didn’t have a stroke.”
She was sick for months. Dobbs also developed neuropathy, muscle weakness and pain, and palpitations.
Something else changed, too: Dobbs started having heavy menstrual cycles due to blood clot formation. She still does.
Dobbs tested positive for having antiphospholipid antibodies, which can attack surrounding cells, causing blood clots.
Microclotting: A Long-COVID and COVID-19 Vaccine Injury Problem
General practitioner Dr. Robin Kerr and cardiovascular researcher Harriet Carroll from Lund University recently published a preprint proposing the hypothesis that blood clotting plays a significant role in long COVID. The authors also wrote that COVID-19 vaccine injury is very similar to long COVID.
Etheresia Pretorius, a distinguished research professor specializing in microclotting at Stellenbosch University, told The Epoch Times that while microclotting may not be the only pathology going on, it is likely a significant contributing factor.
Microclotting can explain many symptoms observed in long COVID and COVID-19 vaccine injury syndrome. Blood clots obstruct blood vessels, impairing the exchange of nutrients. This leads to fatigue, shortness of breath, brain fog, and neuropathy as nearby cells become oxygen-deprived and dysfunctional.
Certain proteins involved in microclotting also play a role in autoimmune and mast cell activation pathways. Microclotting is therefore associated with conditions like autoimmunity and mast cell activation syndrome, characterized by allergic symptoms such as hives, swelling, and difficulty breathing.
Dermatologist Dr. Angela Bowers told The Epoch Times that most of her patients, including both those with long COVID and vaccine-injured individuals, would see some improvement after being given therapies that improve circulation.
The Role of Spike Proteins in Blood Clotting Pathology
Blood clotting pathology has been mentioned in the treatment protocols for both long COVID (pdf) and vaccine injury (pdf) by the Frontline COVID-19 Critical Care Alliance (FLCCC).
The FLCCC doctors and many researchers have noted the spike protein as the primary catalyst for blood clot formation. Spike proteins are found on the surface of the COVID-19 virus, which utilizes them to invade cells and cause harm. Similarly, the mRNA vaccines instruct the body to produce spike proteins.
The spike protein contributes to blood clotting issues through several mechanisms.
Inducing Blood Clot Formation
Spike proteins can trigger clot formation in the blood even without thrombin and platelets.
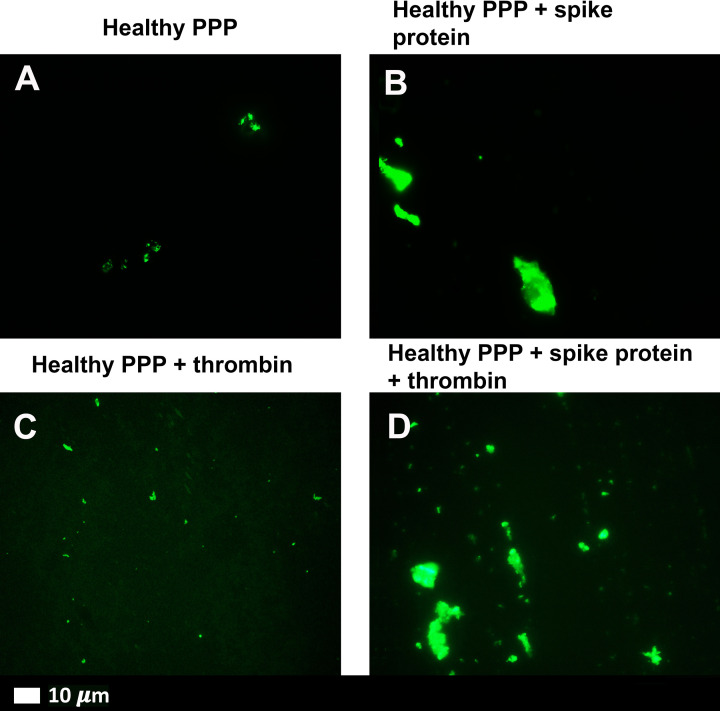
Altering the Structure of Blood Clotting Proteins
Spike proteins change the structure of beta and gamma fibrinogen, complement 3, and prothrombin, resulting in the development of blood clots that are larger and more difficult to break down.
Endotheliitis
Spike proteins damage the inner lining of blood vessels by binding to ACE-2 receptors on endothelial cells. ACE-2 has been identified as the primary receptor used by the SARS-CoV-2 virus for cellular entry, and endothelial cells are abundant in ACE-2 receptors, putting them, particularly, at risk of infection. Consequently, the spike proteins can enter the endothelial cells and activate inflammatory pathways, ultimately leading to blood clot formation.
Platelet Hyperactivation
Spike proteins induce hyperactivated platelets, which have a higher tendency to clump together and adhere to endothelial walls, resulting in the formation of blood clots within blood vessels.
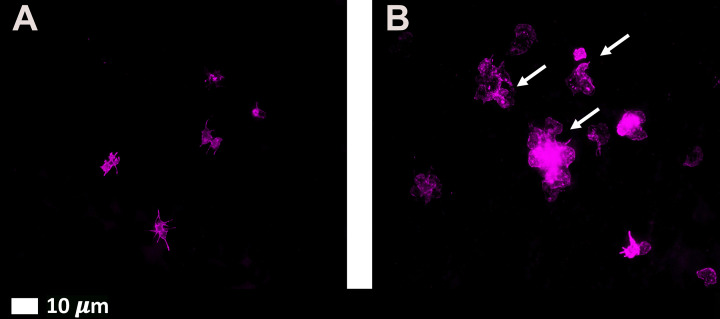 Fluorescence microscopy micrographs of platelets before and after exposure to spike protein. Platelets become activated after being exposed to spike protein. Exhibit A shows normal platelets, and exhibit B shows platelets exposed to spike protein. (Courtesy of Etheresia Pretorius)
Fluorescence microscopy micrographs of platelets before and after exposure to spike protein. Platelets become activated after being exposed to spike protein. Exhibit A shows normal platelets, and exhibit B shows platelets exposed to spike protein. (Courtesy of Etheresia Pretorius)Challenges With Blood Clot Tests
Pretorius, who has researched microclots in diabetes and Alzheimer’s disease, has found that the blood clots formed in acute COVID-19 and long-COVID patients are larger and more resistant to breakdown. They cannot be broken down by trypsin, an enzyme that helps digestion.
Another issue arises when doctors conduct tests to detect blood clots, as the results may return negative.
The most common test involves C-reactive proteins, which are elevated during inflammation, and may suggest blood clot formation. Pretorius’ research found that the insoluble microclots formed in long COVID tend to entrap inflammatory markers such as C-reactive proteins. Since these markers are no longer dissolved in plasma, when doctors take a sample of the plasma solution, the results return as normal.
Another standard test for blood clots checks D-dimer levels. However, D-dimers are only released when blood clots begin to break down. As a result, patients who have blood clots but have not yet broken them down may frequently yield normal test results.
These blood clots create a sludge-like substance in the microvasculature, impeding gas exchange, Dr. Jordan Vaughn, board-certified internist, told The Epoch Times. He compared this to a blocked showerhead, where the pipes may be clean, but the cartridge valves in the head gradually become obstructed over time.
Based in Alabama, Vaughn is one of the leading doctors in the United States studying microclots in long COVID and vaccine injury.
“Just like the piping in your wall doesn’t necessarily shake or make noise until it reaches a critical threshold,” Vaughn said, “a lot of people have accumulation, and that accumulation doesn’t come to the point where it interferes with red blood cell delivery.”
Once gas exchange is hindered or blocked, cells may become dysfunctional due to reduced oxygen, causing symptoms.
Promising Approaches to Address Microclotting
Fortunately, there are several promising approaches to addressing microclotting on the horizon.
Triple Therapy With Anticoagulants and Anti-Platelets
A single anticoagulant or anti-platelet medication may be insufficient to break down spike protein blood clots. Therefore, doctors have combined anticoagulating therapies.
One such combination is known as “triple therapy,” which consists of:
- One daily dose of clopidogrel and aspirin at 75 milligrams
- Two daily doses of apixaban at 5 milligrams
- One daily dose of a proton pump inhibitor
Clopidogrel and aspirin are anti-platelet drugs that prevent platelets from clumping together to form blood clots, while apixaban serves as an anticoagulant.
This therapy was first introduced in December 2021 by a team of researchers that included Pretorius and Professor Douglas Kell, research chair in systems biology at the University of Liverpool (pdf).
A group of 24 long-COVID patients was placed on this regimen for a month, and all patients experienced a significant improvement in symptoms such as brain fog, shortness of breath, poor focus, memory loss, and fatigue. Additionally, microclotting and platelet hyperactivation decreased.
In March 2023, Pretorius and Kell published a second preprint (pdf) regarding 91 patients, most of whom experienced a resolution of symptoms. Despite the high success rate, the authors emphasized that “such a regime must only be followed under expert medical supervision in view of the risk of bleeding.”
During the study, 75 of the 91 participants reported bruising as a side effect. Other side effects were also observed, including minor nosebleeds reported by five patients, increased menstrual bleeding reported by two women, and one patient requiring blood transfusions due to a gastrointestinal bleed.
Nattokinase
Although there haven’t been any clinical trials conducted on nattokinase, health care providers who treat long COVID and vaccine injuries have reported that the supplement appears to be quite effective in breaking down blood clots and relieving patients’ symptoms.
Nattokinase can break down spike proteins as well. As spike proteins are believed to contribute to the formation of abnormal blood clots associated with long COVID and vaccine-related injuries, the additional impact of nattokinase may explain why it is sometimes perceived as beneficial in addressing clotting issues.
Furthermore, nattokinase can reduce the levels of plasminogen activator inhibitor-1 (PAI-1), a substance that promotes the formation of blood clots.
Nurse practitioner Scott Marsland at Leading Edge Clinic intentionally prescribes patients a higher dosage of nattokinase to be taken twice daily by patients experiencing loss of smell and taste. This supplement can effectively assist in breaking down potential blood clots that may obstruct the nerves from receiving sufficient oxygen and nutrients.
Selective Serotonin Reuptake Inhibitors
In their preprint, Kerr and Carroll suggested that selective serotonin reuptake inhibitors (SSRIs) such as sertraline could potentially aid in preventing blood clots.
SSRIs prevent the degradation of serotonin, a neurotransmitter colloquially known as the happiness hormone.
Scientists believe SSRIs can alleviate depression and anxiety by allowing more circulating serotonin to regulate mood and memory. However, studies have also shown that these drugs also have potent anti-inflammatory effects, which may help with mental health.
Sertraline can bind to spike proteins, hindering their interaction with cell ACE-2 receptors. In addition, when combined with aspirin and clopidogrel to treat depression, sertraline has demonstrated anti-platelet and endothelial-protective properties.
Another SSRI included in the FLCCC protocol for treating COVID-19 vaccine injury is fluvoxamine. It effectively reduces inflammation in endothelial cells, thus preventing damage to blood vessels.
Delayed Treatment May Slow Recovery
During an FLCCC conference, Vaughn highlighted that the timeliness of treatment and the patient’s age can influence their response to therapy.As people age, the health of their blood vessels tends to decline, resulting in reduced flexibility and increased vulnerability to changes in blood volume and pressure.
Since endotheliitis can cause blood vessels to age, patients who wait to get treatment may take longer to recover.
The study on 91 patients revealed that individuals experiencing long COVID for less than six months tended to recover more quickly than those with symptoms lasting over six months.
“Delay in treatment may prolong the duration of pharmacotherapy and also increase the likelihood of permanent hypoxic tissue damage. This may manifest as a partial response to treatment or even treatment failure,” the authors wrote.





.png)


Comments
Post a Comment