Streptococcus Salivarius K12 Probiotic Strain Offers New Weapon in Fight Against Infectious Disease and COVID-19
As reported in this short news clip in 2021, research evidence shows that probiotics may help reduce long-haul symptoms after COVID-19. Some people experience symptoms for weeks or months after a COVID-19 infection has resolved. When these symptoms persist for four weeks or more, they are known as long COVID, long-haul COVID, chronic COVID or long-haul syndrome.
The oral probiotic strain Streptococcus salivarius K12 works by crowding out bad bacteria and stopping its ability to colonize. New studies have also found that it has antibacterial and antiviral properties and stimulates inhibitory substances that kill or inhibit the growth of various bacterial species disabling the reproduction of pathogenic bacteria on the oropharyngeal mucus surfaces.
For the majority, current COVID-19 variants present with mild or minor cold or flu-like symptoms including low fever, fatigue, and dry cough. The less common, severe cases present with dyspnea (difficulty breathing) that can rapidly deteriorate into serious complications, such as acute respiratory distress syndrome.
Research has shown that secondary bacterial infections in COVID-19 patients are important causal drivers of mortality outcomes.
A study done in Wuhan, China, published in Frontiers in Microbiology in July 2021, found that bacterial infections (bacteremia and pneumonia) were more common in fatal COVID-19 cases.
The National Institutes of Health noted that in the 1918 Spanish Flu pandemic, upward of 95 percent of mortality was directly attributable to secondary bacterial pneumonia.
Also, during the outbreaks of Severe Acute Respiratory Syndrome (SARS) in 2003 and H1N1 influenza in 2009, bacterial complications were associated with serious outcomes such as death and admission to intensive care. During the 2009 influenza pandemic specifically, almost one in four patients presented with bacterial complications.
A study conducted between April and June 2020, published in Environmental Research and Public Health in February 2022, stated that bacterial colonization increased the length of ICU hospitalization and the mortality rate with 53 percent having bacterial infections among ICU-admitted COVID-19 patients. They advised that clinical management of COVID-19 patients should also consider the assessment of co-infections so that treatment for both COVID-19 and bacterial infection can be administered.
Between January and February 2020, a study was conducted in Wuhan, China, to investigate the benefits of the oropharyngeal probiotic strain Streptococcus salivarius K12 in preventing respiratory tract infections in frontline medical staff who were in close contact with COVID-19 hospitalized patients.
It found that “oropharyngeal probiotic administration significantly reduced the incidence of respiratory tract infections by 64.8 percent, reduced the time experiencing respiratory tract infections and oral ulcer symptoms by 78 percent, shortened the days absent from work by 95.5 percent, and reduced the time under medication where there is no record of antibiotic and anti-viral drug intake in the probiotic group.”
It was also noted that there was no intake of steroid/anti-inflammatory drugs in the probiotic group compared with 10 days of intake of steroid/anti-inflammatory drugs in the control group.
The results of this study indicate that the oropharyngeal probiotic formula containing Streptococcus salivarius K12 “can reduce susceptibility to respiratory tract infections for frontline medical staff fighting against COVID-19.”
Regarding the vaccination status of the enrolled medical staff, only one out of 98 in the probiotic group had the pneumococcal vaccine and four out of 98 had the influenza vaccine, while one out of 95 in the control group had the pneumococcal vaccine and four out of 95 had influenza vaccine. The two groups had no statistically significant differences in vaccination status and outcomes.
The above-mentioned study was published in Frontiers in Bioengineering and Bio Technology in June 2021 and was peer-reviewed by Stephen A. Morse a senior consultant at the Centers for Disease Control and Prevention (CDC) yet the CDC guidelines continue to peddle vaccines and the unapproved investigational medicine Paxlovid.
In May 2022, a study confirmed the intake of the oropharyngeal Streptococcus salivarius K12 as a dietary intervention can effectively reduce episodes of upper respiratory tract infections in school children. Children in the probiotic group experienced 68 percent fewer days of onset of respiratory symptoms than the control group.
The most recent study published in Probiotics and Antimicrobial Proteins in December 2022, found that Streptococcus salivarius K12 evokes an immunological response in the oral cavity—an effect that may contribute to the protection of the host against certain viral infections. The study states, “this is the potential for application as a short-term cross-protective (‘priming’) activity against viral infections initiating within the oral cavity.”
The Oral Microbiome
The importance of our gut microbiome is fairly common knowledge these days—but the lesser-known oral microbiome, also referred to as the oropharyngeal microbiome, may hold the key to virus and bacterial infection prevention.
The oral cavity houses the second largest microbiome in the human body and is made up of distinct communities of bacteria, fungi, viruses, archaea, protists, and other microorganisms, whose compositions are dependent upon environmental conditions that change daily based on multiple factors that we will discuss later in this article.
The association between the microbiome and health has been known and documented in key concepts in ancient medical systems such as Ayurveda and Traditional Chinese Medicine for thousands of years but was first “discovered” in Western science in the 1840s.
In 2007, the Human Microbiome Project began its research to understand the mechanisms governing the similarities and differences in the microbes we share as a population, microbes’ association with diseases, and the functional roles microbiota play in health and disease. The Project’s research has been groundbreaking and is ongoing.
It’s been found that modern practices including the use of antibiotics and vaccines have likely affected the composition and diversity of the human microbiome and more studies are underway to discover how to leverage and utilize natural microorganisms to combat viruses and chronic illness.
The oral microbiome contains one of the most diverse and unique communities of microbes in the human body, yet this niche was relatively understudied as compared to the gut microbiome. As of 2020, a PubMed search with “oral microbiome” resulted in 746 articles, as compared to 5605 with “gut microbiome.”
In the last two years, research on the oral microbiome has dramatically increased. A PubMed search of “oral microbiome” at the time of writing this article resulted in 102,461 articles compared to 108,688 “gut microbiome” articles.
According to the expanded Human Oral Microbiome Database, only 58 percent of the oral bacterial species have been officially named, 16 percent have been cultivated yet remain unnamed, and 26 percent are uncultivated.
COVID-19 and the Oral Microbiome
A growing number of studies show associations between diseases and viruses and changes in the oral microbiome. For example imbalances in the oral microbiome can cause gut microbes to produce carcinogenic toxins triggering gut inflammation and metabolic problems.
The mouth is an entry point to the respiratory and digestive systems and is highly vascularized. High vascularization of the mouth can contribute to illness by exacerbating the inflammatory response causing vascular changes, and leading to hypoxia. This can lead to oral changes associated with endocrine illness, as well as an increased risk of cardiovascular disease, clogged arteries, stroke, and peripheral vascular disease.
Replication of COVID-19 occurs in the nose and throat and initially develops as a respiratory infection in the cells of the nasal cavity and respiratory tract of which 95.86 percent are expressed in cells that cover the surface of the tongue (epithelial cells).
A study published in the journal Nature revealed that COVID-19 might interact with members of the oral microbiome in either the lungs or the oral cavity. Viral shedding from the nose and mouth is also a major factor in transmission with evidence suggesting that the first responses in this battleground may help determine who will develop severe disease.
Studies show that lung microbiota is more similar to those in the oropharynx than those in the nasopharynx or lower digestive tract. Because of this, the oral microbiome could be a driving force in regulating the immune system in the mouth, which could impact the ability of pathogens to cause infection.
Germ Warfare
Not all microbial species like each other or get along. Some live in harmony together and others engage in relentless war. Bacterial competition in the microbiome is driven by the fight for resources, such as space, light, and nutrients.
Leveraging this competitive behavior in bacteria by colonizing our oral microbiome with good bacteria—bacteria that are beneficial to the body and deliver a health benefit—is one strategy microbiologists are finding has potential in virus and infection prevention.
Good bacteria—some of which have been developed as probiotics—are also a good source of antimicrobial molecules that are part of the innate immune response and whose fundamental biological role is to control pathogenic microorganisms, including negative bacteria, fungi, and viruses.
Antimicrobial molecules are produced as a first line of defense against invading pathogens and form a foundation for the development of new therapeutics.
Scientific Breakthroughs
Streptococcus salivarius K12 which has been developed as a probiotic for the oral cavity has been clinically demonstrated to improve the upper respiratory tract microbiota protecting the host from pathogenic bacteria, fungi, and viruses, reducing the incidence of viral respiratory tract infections and bacterial co-infections.
There are currently over one hundred studies conducted to date on its benefits and more are underway.
In the study published in Frontiers in Bioengineering and Bio Technology in June 2021, whose primary objective was to investigate the benefits of Streptococcus salivarius K12 probiotic in preventing respiratory tract infections in frontline medical staff who were in close contact with COVID-19 patients, found that the clinical benefit of oropharyngeal probiotic plays a role in creating a stable upper respiratory tract microbiota capable of protecting the host from respiratory tract infections. The authors state:
“Its anti-viral capability to build a well-established first-line defense on the upper respiratory tract and oropharyngeal microbiome to protect individuals from respiratory tract infection could be a promising strategy to prevent respiratory tract infections, including COVID-19 infections.”
Streptococcus salivarius K12 works in several ways to fight off viral infections in its host.
One of the ways it works is by producing various salivaricins which can inhibit the growth of various pathogens, including bacteria, by binding to their surface and disrupting their cell membranes. This can help to prevent viral infections from taking hold in the body and shows promising inhibitory activity towards an array of bacterial pathogens.
Streptococcus salivarius K12 also stimulates an anti-inflammatory response, actively protecting the host against inflammation and cellular death caused by pathogens. It promotes cellular health and homeostasis and may protect host tissues from damage caused by other immunostimulatory cells and products.
Additionally, Streptococcus salivarius K12 can also compete with pathogenic bacteria for space and nutrients in the host’s oral cavity which prevents the colonization of viruses and other pathogens.
A recent study published in December 2022 in Probiotics and Antimicrobial Proteins investigated whether the ingestion and oral cavity colonization by Streptococcus salivarius K12 is associated with the enhancement of IFN-γ (a protein that affects the immune system) levels in saliva.
The study found that the oral probiotic strain did increase IFN-γ levels in human saliva within 24 hours of consumption—an effect that may contribute to the protection of the host against certain virus infections.
The increase in IFN-γ levels in the oral cavity helps to fight off viruses in several ways:
- Activation of other immune cells: IFN-γ activates immune cells such as macrophages and natural killer cells, which can directly kill virus-infected cells.
- Induction of antiviral genes: IFN-γ can also induce the expression of genes that produce proteins that inhibit viral replication and spread.
- Enhancement of adaptive immune response: IFN-γ activates other T cells, such as CD4+ T cells and CD8+ T cells, which play a critical role in the adaptive immune response against the virus.
Keeping Our Microbiome Strong
Streptococcus salivarius K12 naturally occurs in the mouth and throat of healthy adults and children. But the oral microbiome composition can be highly variable between individuals in different states of health, with lifestyle, age, and environment playing key roles and is constantly changing due to multiple factors.
Several things that can harm the status of the oral microbiome are smoking, alcohol consumption, hormonal changes, poor diet, and spicy foods.
The K12 strain became the first of Streptococcus salivarius species to be commercially developed as a probiotic and many high-quality brands on the market include this probiotic strain. Here are two notable brands that have been studied and published in the randomized control trials mentioned in this article:
- Bactoblis oropharyngeal probiotic formula by Probionet GmbH was used in the 2020 clinical trial done with COVID-19 frontline medical staff. There are 31 clinical trials conducted with Bactoblis to date, and 10 review or meta-analysis articles mentioning the studies conducted with Bactoblis.
- Blis Probiotics, formulated by BLIS Technologies Ltd, a New Zealand-based company, has completed multiple studies on their product. Published studies on BLIS probiotics explore its anti-inflammatory immune effects and its efficacy as an oropharyngeal probiotic. Their most recent clinical trial shows protection of the host against certain virus infections.
Probiotics for COVID-19 Studies 2023
Tips on Buying and Taking Oral Probiotics
When buying oral probiotics, there are several things to look for to ensure that you are getting a high-quality product. I spoke to John Hale who has a doctorate in microbiology and has been involved in numerous studies, clinical trials, and research on Streptococcus salivarius K12. He shared some great tips to keep in mind when purchasing a quality oral probiotic:
- “One important thing to look for is if the company is a member of the International Probiotics Association (IPA). Members of the IPA must adhere to guidelines, so you can trust that they are producing safe and effective products,” Hale said. You can check if a company is a member of the IPA by looking for the IPA logo on the packaging or checking the IPA’s website.
- Look at the name of the bacteria that is listed on the packaging. “It’s important to see the genus, species, and strain name of the bacteria. For example, when buying this specific oral probiotic strain you want to see ‘Streptococcus salivarius K12.’ This ensures that the company has done research and understands what they are giving you,” he said.
- “Finally, the amount of bacteria listed on the packaging is usually not as important as the dose that was found to work in clinical trials and studies. It is important to note that more is not always better. The specific dose that was found to work in the trials and studies on Streptococcus salivarius K12 is one billion CFU [colony-forming unit] or greater,” he said.
How often should we take oral probiotics for viral and respiratory tract infection defense?
Hale says, “for upper respiratory infection prevention, you should take a daily dose during peak periods when you are likely to get sick—like the winter months for example. It is also important to take them daily around times you will be in a confined environment with other people.”
Superbugs, Vaccines, and the Antibiotic Apocalypse
Anti-microbial resistance (AMR) is a global public health crisis that has dramatically increased over the past decade.
The overuse of antibiotics and vaccines can lead to the development of “superbugs”—strains of bacteria that have evolved to resist the effects of both antibiotics and vaccines. As these superbugs spread, they can become more difficult to treat and can lead to an “antibiotic apocalypse” in which common infections can become deadly.
A review done on AMR that began in 2014 and published in 2016, estimated that by 2050 as many as 10 million people could die each year as a result of AMR.
Many felt this number was inflated because at the time there were about 700,000 deaths per year associated with AMR—but a global analysis of AMR published in The Lancet in January 2022, estimated that 4.95 million deaths were associated with AMR in 2019.
Fortunately, many types of bacteria that live on and in our bodies that are harmless to us produce bacteriocins that may be of great value in the development of new and novel antibacterial therapies in this era of emerging antibiotic resistance.
Administration of the slow-dissolving oropharyngeal probiotic strain Streptococcus salivarius K12 is a promising strategy to protect individuals during the outbreak of seasonal or emerging respiratory infectious diseases as well as mitigating the worldwide public health crisis related to the development of pathogenic antibiotic-resistant strains that caused respiratory infections.
References:
https://pubmed.ncbi.nlm.nih.gov/34003804/
https://www.nature.com/articles/s41385-020-00359-2
https://www.chop.edu/news/feature-article-viral-shedding-and-covid-19-what-can-and-can-t-happen
https://www.cell.com/cell/fulltext/S0092-8674(21)00882-5
https://www.frontiersin.org/articles/10.3389/fbioe.2021.646184/full
https://link.springer.com/article/10.1007/s12602-022-10010-0#citeas
https://link.springer.com/article/10.1007/s12602-021-09822-3
https://www.jci.org/articles/view/11918
https://www.frontiersin.org/articles/10.3389/fnut.2022.900448/full
https://www.liebertpub.com/doi/10.1089/jir.2012.0116
https://www.sciencedirect.com/science/article/pii/S2452231719300144
https://www.britannica.com/science/human-microbiome
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7559905/
https://link.springer.com/article/10.1007/s12038-019-9939-6
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7188910/
https://www.nature.com/articles/nature06244
https://www.microbiologyresearch.org/content/journal/micro/10.1099/mic.0.043174-0
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074908/#B3-microorganisms-08-00308
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074908/#B4-microorganisms-08-00308
https://www.ncbi.nlm.nih.gov/books/NBK545271/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879262/
https://www.pharmextracta.com/en/product/bactoblis-30-tablets/
https://www.frontiersin.org/articles/10.3389/fmicb.2021.682571/full
https://www.frontiersin.org/articles/10.3389/fmicb.2021.682571/full#B20
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2519405/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7074908/
https://www.nature.com/articles/s41368-022-00163-7
https://pubmed.ncbi.nlm.nih.gov/25604788/
xhttps://pubmed.ncbi.nlm.nih.gov/30526505/
https://link.springer.com/chapter/10.1007/978-981-15-4814-7_6
https://scholar.google.com/scholar_lookup?title=High+expression+of+ACE2+receptor+of+2019-nCoV+on+the+epithelial+cells+of+oral+mucosa%2E&journal=Int%2E+J%2E+Oral+Sci%2E&author=Xu+H.&author=Zhong+L.&author=Deng+J.&author=Peng+J.&author=Dan+H.&author=Zeng+X.&publication_year=2020&volume=12&pages=1%E2%80%935
https://www.sciencedirect.com/science/article/pii/S0960982215014098
https://pubmed.ncbi.nlm.nih.gov/30111628/
https://pubmed.ncbi.nlm.nih.gov/23041248/
https://www.pharmextracta.com/en/product/bactoblis-30-tablets/
https://link.springer.com/article/10.1007/s12602-010-9045-4
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02724-0/fulltext
https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
https://doaj.org/article/d826c6a59c3142ed9344c22a90f4f883
https://www.mdpi.com/2076-2607/10/10/1926
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9012604/
https://link.springer.com/article/10.1007/s12602-021-09822-3
https://www.mdpi.com/2072-6643/13/12/4392
https://www.cell.com/trends/microbiology/fulltext/S0966-842X(20)30058-5
About the Author: Christy A. Prais received her business degree from Florida International University. She is founder and host of Discovering True Health, a YouTube Channel and podcast dedicated to health and wellness, and contributing journalist for the Epoch Times. Christy also serves on the advisory board at the Fostering Care Healing School.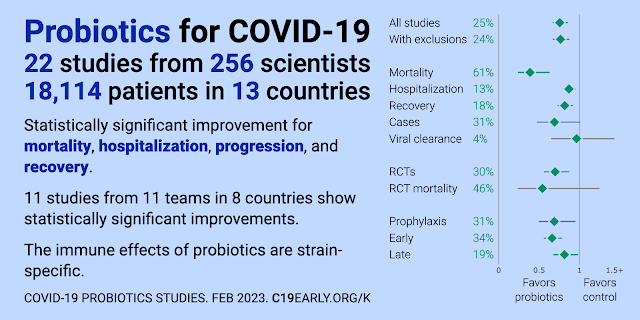






.png)

Comments
Post a Comment