Heart Inflammation and COVID-19 Vaccines: Special Report by The Epoch Times
Data from the clinical trials of the Novavax COVID-19 Vaccine and global vaccine safety monitoring systems suggest an increased risk of myocarditis and pericarditis following Novavax vaccination. Data from post-authorization monitoring of Janssen COVID-19 Vaccine (Johnson & Johnson) suggest a possible increased risk of myocarditis and pericarditis following Janssen vaccination.
Cases Started in January 2021
Doctors started seeing postvaccination myocarditis cases in January 2021, months before the public was informed about them. The U.S. military researchers, for instance, detailed in a paper that 22 previously healthy service members suffered from myocarditis after vaccination as early as January 2021.Dr. Dror Mevorach, a co-author of the paper, said he tried warning Pfizer of the possible link between myocarditis and its shot.
For many cases, doctors ruled out other possible causes, such as COVID-19—strengthening support for the theory that the vaccines were the cause.
JAMA Pediatrics did not publish any studies about myocarditis after COVID-19 vaccination until Feb. 25, 2022. A query to Dr. Christakis returned an away message.
The number of cases reported in journals increased as 2021 went on, following the pattern of VAERS reports.
The first cases were reported in the literature in March 2021. Just 16 more were published by the end of June 2021. The number spiked to 290 in July 2021.
The Israeli group's report was not released until October 2021, more than six months after the first case was detected.
"It takes time to process the data and collect it and report on it. Of course, when you write an article, there is also a review and a revision process, and it took several months until we were able to publish it online," Dr. Rabea Asleh, director of the heart failure unit and the cardiovascular research center at the Hadassah University Medical Center and a co-author of the paper, told The Epoch Times.
He added later, "There were early reports after the vaccine of myocarditis, we were just waiting to provide more comprehensive information."
'Seems Reasonable'
A case report from Spanish researchers was the first in the world to be published in the literature. Dr. Javier Bautista Garcia and colleagues reported a 39-year-old doctor who was hospitalized for six days following his first dose of a Pfizer vaccine.The doctor tested negative for COVID-19, and researchers excluded other possible triggers.
"Given the evident temporal relationship and the serological pattern compatible with immunization after the vaccine, having ruled out an acute infection, it seems reasonable to associate the clinical picture developed by this patient with an adverse reaction to the BNT162b2 vaccine against COVID-19," the researchers wrote. BNT162b2 is the name for Pfizer's shot.
"Even if no causal relationship can be demonstrated in this case report between the second dose of BNT162b2 mRNA COVID-19 vaccination and acute myocarditis, the timing of the onset and the inflammatory nature of the event make the relationship plausible," they said.
 The explosion of reports in June 2021 included a case series of American children as young as 14 suffering from myocarditis
shortly after receiving shots and requiring hospitalization. U.S.
researchers also reported four cases in Pennsylvania, one case in New Jersey, six cases in Massachusetts, four cases in Minnesota, seven cases in Texas and Virginia, another case in Texas, one case in California, two cases in Missouri, one case in New York, two cases in Hawaii, and seven cases in North Carolina.
The explosion of reports in June 2021 included a case series of American children as young as 14 suffering from myocarditis
shortly after receiving shots and requiring hospitalization. U.S.
researchers also reported four cases in Pennsylvania, one case in New Jersey, six cases in Massachusetts, four cases in Minnesota, seven cases in Texas and Virginia, another case in Texas, one case in California, two cases in Missouri, one case in New York, two cases in Hawaii, and seven cases in North Carolina.
First Acknowledgement
Israeli media reported in April 2021 a second post-vaccination myocarditis death, a 35-year-old previously healthy young man. Officials there, meanwhile, revealed that an investigation determined 55 myocarditis cases were in relation to vaccination and 81 more were probably related."We found a high and statistically significant risk of myocarditis ... and in our opinion the relationship found can fulfill the criteria for a causal relationship," the experts said. The ministry said: "The conclusion is that there is a high probability of a connection between the administration of the second Pfizer vaccine and the increased risk of myocarditis, especially in young boys."
The CDC also formed a new team, drawing from across the agency, in May 2021. It focused on digging into reports of myocarditis in the United States. And the agency held calls for health care providers, including doctors from pediatric hospitals, to craft guidance and give advice. But it shielded much of the work from public view, including not discussing myocarditis at all during open meetings with vaccine advisers on May 5, 2021, and May 14, 2021.
Ms. Ekanayake, Aiden's mother, had read the Israeli research. But she and her son decided he should get vaccinated because they believed the benefits outweighed the risks, in part because of Dr. Walensky's public statement.
"She said at some point that there wasn't a link, so we proceeded," Ms. Ekanayake told The Epoch Times. "So when my son was vaccinated, there was absolutely no informed consent."
Aiden received his first shot on May 10, 2021.
Kyle Warner, a professional mountain bike racer who lives in the Western U.S. and suffered myocarditis after being vaccinated in May and June 2021, said the lack of warning from U.S. officials about the possible adverse event also led to him receiving a vaccine.
"A lot of us were under the false assumption that 'well, it sounds like no one's been hurt so far, there hasn't really been any issues going on that I've heard of, so it seems to be safe and effective, like they're saying,'" Mr. Warner said. "It kind of perpetuated this myth of 'safe and effective, period.'"
If a warning was issued, "it would have saved me," he added.
Risk-Benefit Analyses
COVID-19 poses little risk to young, healthy people. That means there's a higher bar of effectiveness and safety vaccines must pass for vaccination to make sense, experts say.No other suspected or confirmed side effects, such as severe allergic shock, were included as a risk.
The same number of doses would prevent thousands of COVID-19 cases, hundreds of hospitalizations, and a handful of deaths over 120 days, the agency estimated.
"The direct benefit-risk assessment shows positive balance for all age and sex groups," Megan Wallace, a CDC official, told the meeting.
The foundations of the assessment were questioned by some researchers, who said that if just one assumption in the model were changed, the calculus shifted to risks outweighing benefits.
The researchers improved on the CDC's model by subtracting incidental hospitalizations and stratifying by underlying conditions, making it possible to make one set of calculations for healthy young people and another for those with poor health.
The researchers said officials should study the issue more closely and consider following the example of other countries such as Sweden that were holding off on recommending the vaccination of healthy children.
Dr. Tracy Beth Hoeg, one of the researchers, told The Epoch Times that she thought U.S. authorities would make changes due to the research. The main options: pause the vaccinations in children, lower the recommended number of doses from two to one, or rescind the recommendations for healthy kids.
Calls for Pause
While the CDC maintained that all people aged 12 and older, regardless of health and other factors, should get vaccinated, a growing number of doctors and other experts voiced opposition to the one-size-fits-all approach.That included Margery Smelkinson, an infectious disease expert with the U.S. National Institutes of Health's National Institute of Allergy and Infectious Diseases.
Some other doctors and health authorities, though, sided with the CDC.
"The fact that you have a case where there may be a link to the vaccine, and at the same time there are tens of thousands of other vaccinees that ended up being safe, this does not justify a pause," Dr. Ofer Habakkuk, director of the cardiology hospitalization department at Tel Aviv Sourasky Medical Center in Israel, told The Epoch Times.
"We were calling for a moratorium until a study was done," Dr. Bostom told The Epoch Times.
"I think right then we should have done trials with young healthy folks," Dr. Koka. "There would have been equipoise, meaning there were enough parents that would have said 'okay, go ahead, we don't know, so we will allow our kids to be randomized to see what the side effect profile is.'"
No U.S. researchers appeared to finish prospective studies before 2023. The U.S. National Institute of Allergy and Infectious Diseases, which would be the most likely institution to fund prospective studies and was led until late 2022 by Dr. Anthony Fauci, did not respond to a request for comment.
Undercutting the Narrative
Only a small number of prospective studies have been completed to date.Other researchers performed prospective studies among vaccinated people before and after a booster.
The CDC initially said 62.8 cases per million second doses were reported among male adolescents. Later in 2021, two sets of Israeli researchers reported 107 to 136 cases per million second doses among young males.
The FDA said in late 2021 that the excess risk of myocarditis among males aged 16 or 17 approached 200 cases per million shots. A group of U.S. researchers soon after pegged the incidence as 195.4 cases per million second doses among males aged 12 to 39. They found patients that were "overlooked" by CDC researchers.
The CDC in early 2022 reported that the rate of reported cases had climbed to 105.8 per million second doses among males aged 16 or 17 while Israeli officials reported rates as high as 153 cases per million second doses among males aged 16 to 19.
 Nordic researchers soon after reported excess rates as high as 274 per
million among young males who received two shots.
Nordic researchers soon after reported excess rates as high as 274 per
million among young males who received two shots.
Some researchers grouped cases by vaccine type and discovered Moderna recipients were more likely to experience myocarditis.
Canadian researchers, for instance, found that excess cases after a second dose were 162 per million for young, male Moderna recipients, compared to 31 per million for Pfizer recipients in the same population. Cases were also higher among women who received Moderna's shot.
Researchers in England found 14 excess cases per million second Pfizer doses among males under 40, and 97 excess cases after second Moderna doses among the same population.
As Risk Grows, Benefits Shrink
The observational data, case reports, and prospective studies added more clarity to the myocarditis risk. It was more common than originally thought and a problem that didn't always resolve quickly. The vaccines, meanwhile, have performed worse against newer variants.After Dr. Hoeg's paper, the FDA found that in one of six modeled scenarios, the projected number of myocarditis and pericarditis cases caused by Pfizer's vaccine would exceed COVID-19 hospitalizations and deaths among 5- to 11-year-olds. The outlier scenario projected 156 excess myocarditis hospitalizations, compared to just 21 prevented COVID hospitalizations, among males aged 5 to 11, and 28 excess myocarditis hospitalizations compared to 21 prevented COVID hospitalizations among females of the same age group.
The presentation resulted in the agency authorizing, and the CDC recommending, Pfizer's shot for the age group.
Another FDA analysis in the spring of 2022, projecting at least 80 percent effectiveness against hospitalization—an outdated figure—estimated COVID-19 vaccines would cause more myocarditis cases than prevented hospitalizations in males aged 16 or 17 in two of three scenarios. The agency, as usual, did not stratify by underlying health or prior infection, and did not include any risks outside of myocarditis.
The methods they used drew widespread scrutiny, including how they only studied people with an official COVID-19 diagnosis. That left out children who were tested at home or not tested due to mild symptoms.
"A lot of parents were reaching out to us as a group, saying, 'hey, I've looked through the scientific literature ... I'm worried about my young, healthy, athletic son, getting myocarditis or suffering some other adverse event,'" Kevin Bardosh, one of the researchers, told The Epoch Times.
"You always find out more risks down the road," Dr. Hoeg said. "And then of course we learned that the benefits didn't last as long as anyone seemed to think they would."
The CDC has defended its risk-benefit estimates.
Boosters Rolled Out
Authorities initially promoted a primary series, or two shots of the Moderna or Pfizer vaccine, as protective, with no indication of future doses.Data emerged in mid-2021 that the vaccine-bestowed protection was eroding over time.
The FDA on Sept. 22, 2021, authorized a Pfizer booster for many Americans 18 and older to try to restore some of the lost protection. It relied on data from a trial of just 306 people, among whom no cases of myocarditis were reported. No efficacy data was provided.
The FDA said that the risk of myocarditis after booster shots was not known while Israeli authorities said they already had a case of myocarditis after starting the booster earlier that fall. One person in Pfizer's booster trial suffered a heart attack, one symptom of myocarditis.
CDC officials recommended the booster for certain populations. They projected one million boosters would cause up to 26 myocarditis cases in the 18- to 29-year-old population while preventing up to 114 hospitalizations and as few as zero hospitalizations. They estimated that 8,738 people in the population would need a booster to prevent a single hospitalization.
Booster availability was later expanded to all populations aged 5 and older, including 16- and 17-year-olds, by inferring effectiveness and safety from an adult population. There was no mention of myocarditis. Moderna's booster was also authorized and recommended based on a trial that included a case of pericarditis. Dr. Walensky said the COVID-19 vaccines "are safe and effective."
Ben Cutler, a Boston resident, was among those who received a booster after the clearance and recommendation. He received a Moderna booster on Dec. 14, 2021. He was 26.
Mr. Cutler suffered the worst pain of his life and required hospital care. He was diagnosed with myopericarditis from the shot.
While other countries took a cautious approach with the booster, "for some reason in the U.S. they just want to give it to everyone without any real data to show that it has any benefit," Mr. Cutler told The Epoch Times.
Long-Term Problems and Deaths
Vaccine-induced myocarditis has caused both long-term problems and deaths, researchers have confirmed.A number of other papers have identified persistence of symptoms, inflammation, edema, and/or LGE.
Deaths, meanwhile, began being reported in early 2021.
The certifier who examined 24-year-old George Watts Jr., a college student in New York, wrote on Mr. Watts' death certificate that the death was due to "COVID-19 vaccine-related myocarditis."
Joseph Keating, 26, of South Dakota, died from myocarditis, according to his death certificate, with his recent Pfizer booster shot listed as contributing.
Mr. Keating’s sister Kaylee Koch, told The Epoch Times that the family tried contacting the CDC but have never received a response. The CDC did not respond to a request for comment.
Other young people have died shortly after COVID-19 vaccination with causes similar to or related to myocarditis. Ernest Ramirez Jr., a 16-year-old in Texas who was pronounced dead after collapsing five days following a Pfizer jab, died from an enlarged heart, according to his autopsy. Myocarditis can cause an enlarged heart.
Researchers in other countries have also confirmed deaths caused by myocarditis following vaccination.
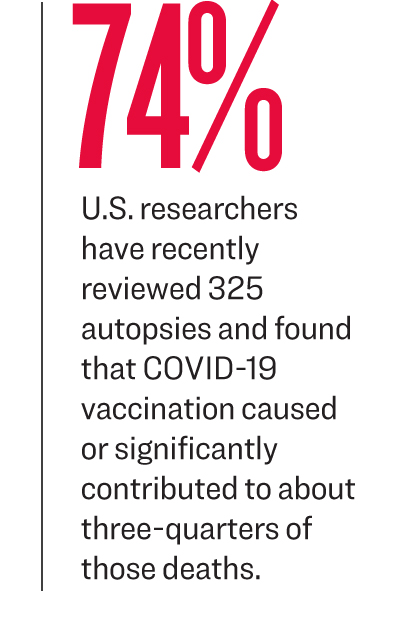 U.S. researchers who recently reviewed 325 autopsies found in a preprint paper that COVID-19 vaccination caused or
contributed to about three-quarters of those deaths, including nine
involving myocarditis.
U.S. researchers who recently reviewed 325 autopsies found in a preprint paper that COVID-19 vaccination caused or
contributed to about three-quarters of those deaths, including nine
involving myocarditis.
Myocarditis and COVID-19 Vaccines: How the CDC Missed a Safety Signal and Hid a Warning", from The Epoch Times.
- Timeline: COVID-19 Vaccines and Myocarditis
- Resource: Studies on Myocarditis and COVID-19 Vaccination
Therapeutic Adjuncts for Vaccine induced myocarditis/pericarditis
- ACE inhibitor/ARB, together with carvedilol as tolerated to prevent/limit progressive decline in cardiac function.
- Colchicine in patients with pericarditis – 0.6 mg/day orally; increase to 0.6 mg twice daily if required. Reduce dose if patients develop diarrhea. Monitor white blood cell count. Decrease dose with renal impairment.
- Magnesium to reduce the risk of serious arrhythmias (A starting dose of 100 to 200 mg daily is suggested, increasing the dose as tolerated up to 300 mg (females) to 400 mg daily).
- Coenzyme Q (CoQ) 200-400mg/day. (R, R, R)
- Omega-3 fatty acids – EPA/DHA 2-4 g/day (R). Increase dose slowly as tolerated. Resveratrol/flavanoid combination for its anti-inflammatory and antioxidant properties.
- Referral to a cardiologist or ER in case of persistent chest pain or other signs and symptoms of cardiac events are observed.
Resources for Those Injured by the COVID Jab
So, the primary task to prevent and/or address post-jab injuries is to eliminate the spike protein. Ivermectin and hydroxychloroquine bind to and facilitate the removal of spike protein. According to McCullough, nattokinase, bromelain and curcumin also help degrade the spike protein.
The World Health Council has also published lists of remedies that can help inhibit, neutralize and eliminate spike protein, which most experts agree is the primary culprit. See, “World Council for Health Reveals Spike Protein Detox.”






.png)

Comments
Post a Comment