New diabetes post-acute Covid (PASC, Long Covid), an inconvenient truth
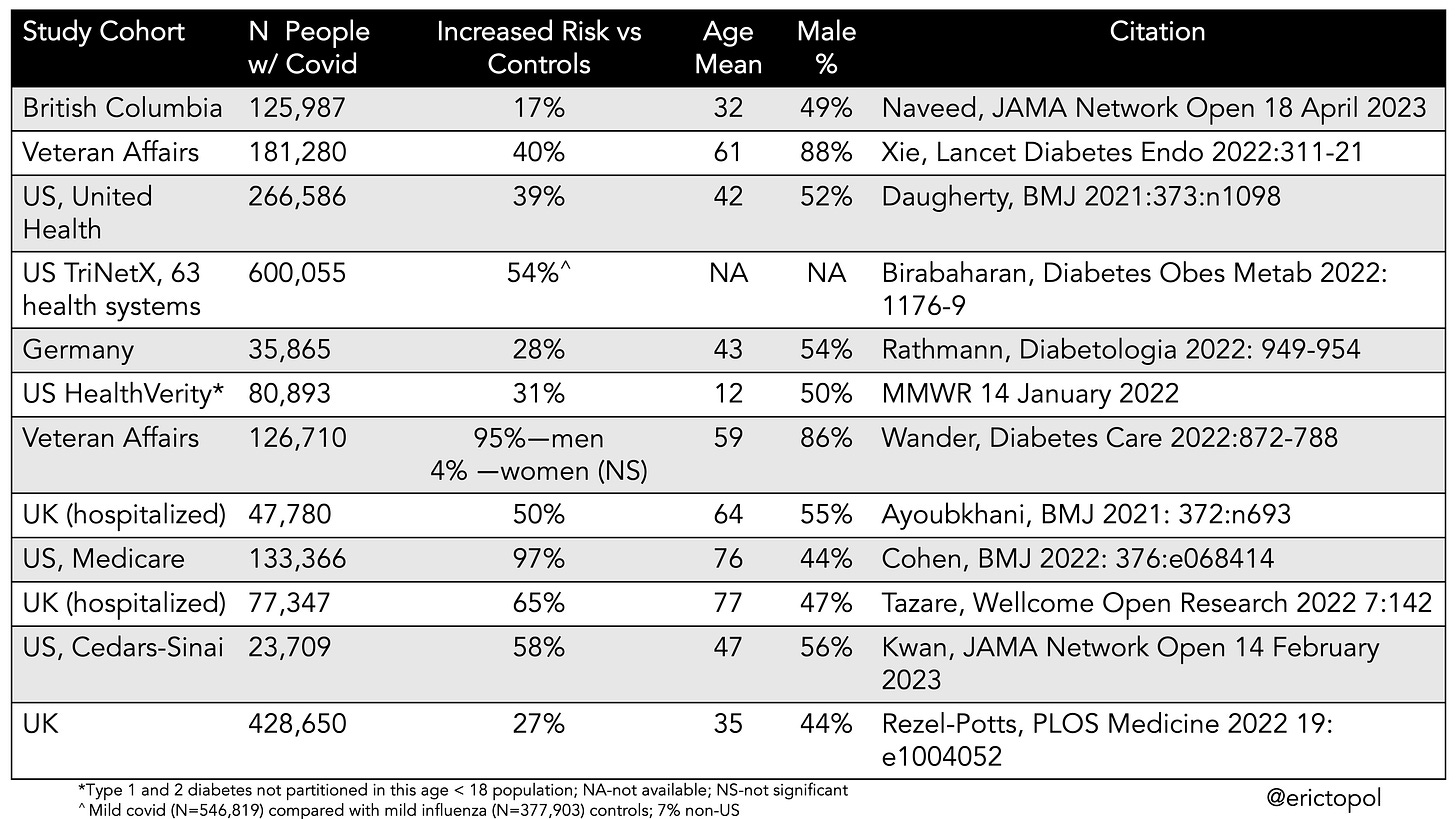
Back in 2020, when the first signals that diabetes (particularly Type 2) was showing up more than a month out from Covid, the hope was that it was spurious. But then study after study have been confirming this sequela. A new study (JAMA 2023) from the province of British Columbia in Canada, a comprehensive population assessment, is the 12th report of a heightened risk of Type 2 diabetes after Covid. This cohort was younger than most (mean age 32 years) and the increased risk of 17% was less than most of the previous publications. I’ve summarized the key data for Type 2 diabetes risk in the Table below. As you can see, the cohorts varied considerably with respect to age and sex. Consistently, the risk has been seen to be higher in men, in people of advanced age, and among those obese, and those with severe Covid. The increased risk compared with controls ranges widely from 17% to 97% with only 1 of these studies not showing a significant increase in a subgroup (among women in a Veteran Affairs report). The CDC published a MMWR on a cohort less than age 18 which did not partition Type 1 and 2 diabetes, but overall showed a 31% increased risk.
Indeed, a previous systematic analysis reported an increased risk for either Type 1 or 2 diabetes after Covid in 12 of 14 studies, ranging from 11% to 276%. That was prior to some of the newer publications included in the Table below.
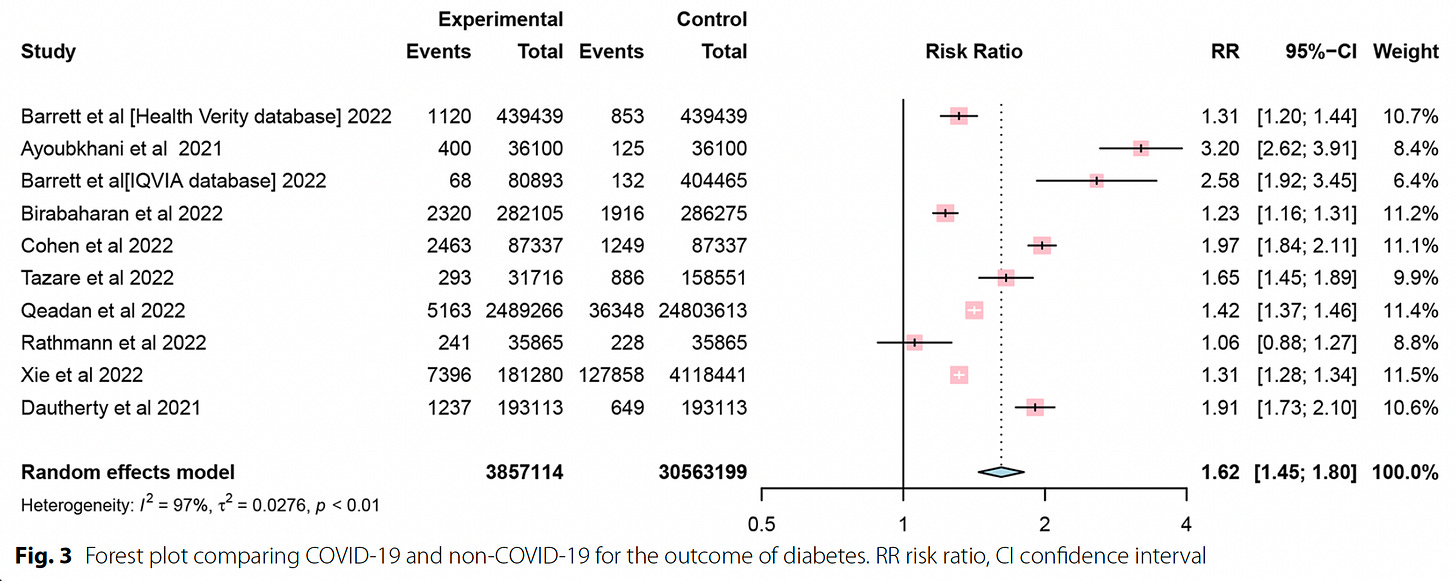
There have been a few meta-analyses of the data to quantify the increased risk, such as shown in the graphic below, with an overall 62% increased risk of diabetes (Type 1 or 2) or another coming up with a 66% increased risk. With the new studies aggregated since these systematic meta-analyses, the risk is about 50% increased, which, at the population level, corresponds to at least an absolute 3 to 5% excess burden of diabetes.
One recent report highlighted the potential protective effect of vaccination before infection with a 37% reduction of incident diabetes following Covid. Whether the incident diabetes and glucose dysregulation are long-term is not yet resolved, with some reports suggesting that it can improve or even fully resolve over time. The large VA study suggested that multiple reinfections raise the risk of Type 2 diabetes.
The reports for higher risk of Type 1 diabetes in children have been less consistent, with studies from Denmark and Canada that did not show heightened risk. Nevertheless, there are multiple studies that have supported a higher risk for this form of autoimmune diabetes, such as a large report from over 27 million people in the United States (42% increase) or from Norway (60% increase), in addition to Scottish and German registries and others.
What is the Mechanism?
We know that SARS-CoV-2 has tropism for organs throughout the body, and the pancreatic β-islet cells, which express entry factors ACE2 and TMPRSS2, have been demonstrated to be infected by the virus in cultures of human islets and in post-mortem studies.
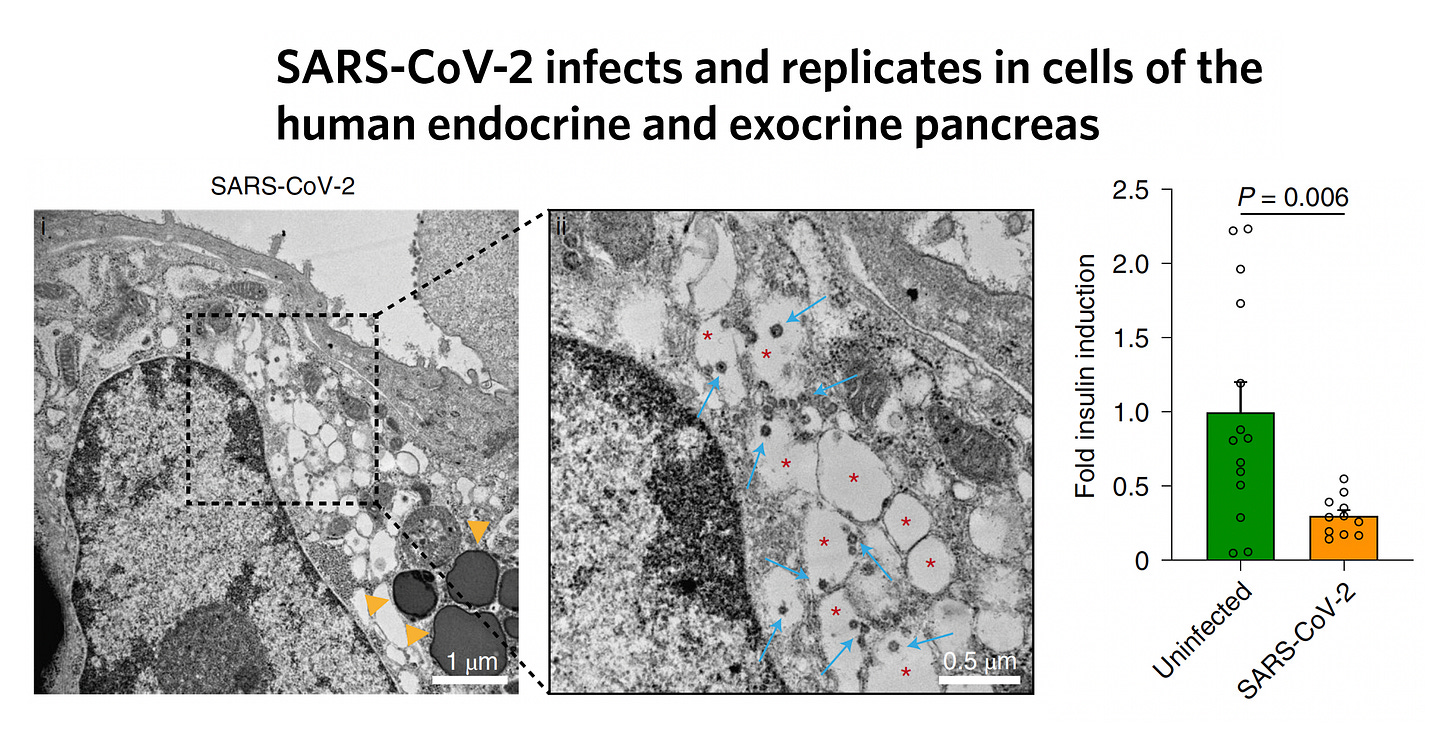
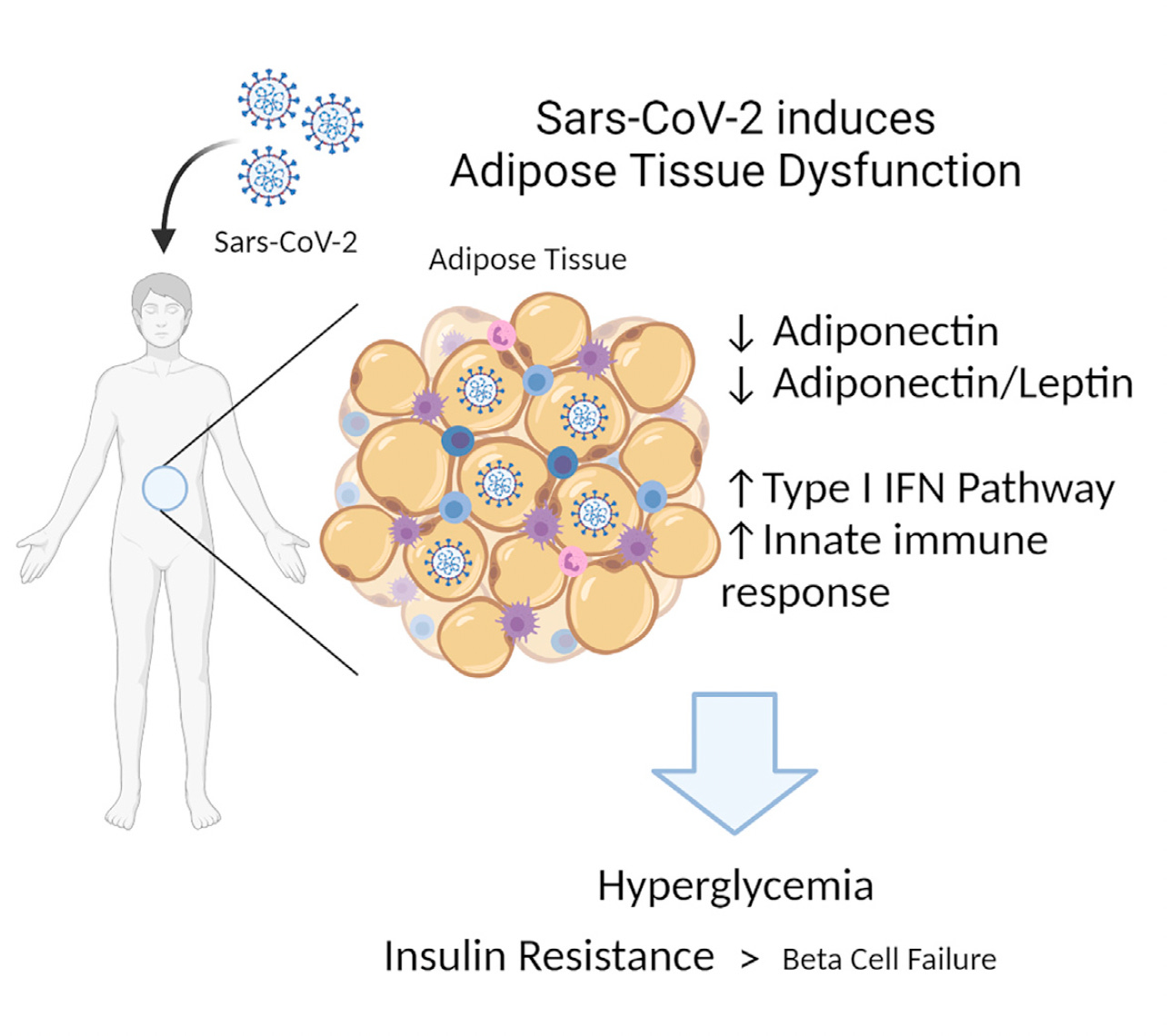
But direct infection of β-islets with cell damage or death is not requisite for developing diabetes. It can occur from inflammation of the islets or the infection may promote β-cell exhaustion. Beyond that, the role of the liver or adipose tissue in pathogenesis is unknown. When fat tissue is infected, there is less adiponectin secreted, which would confer insulin resistance, as schematically shown below.
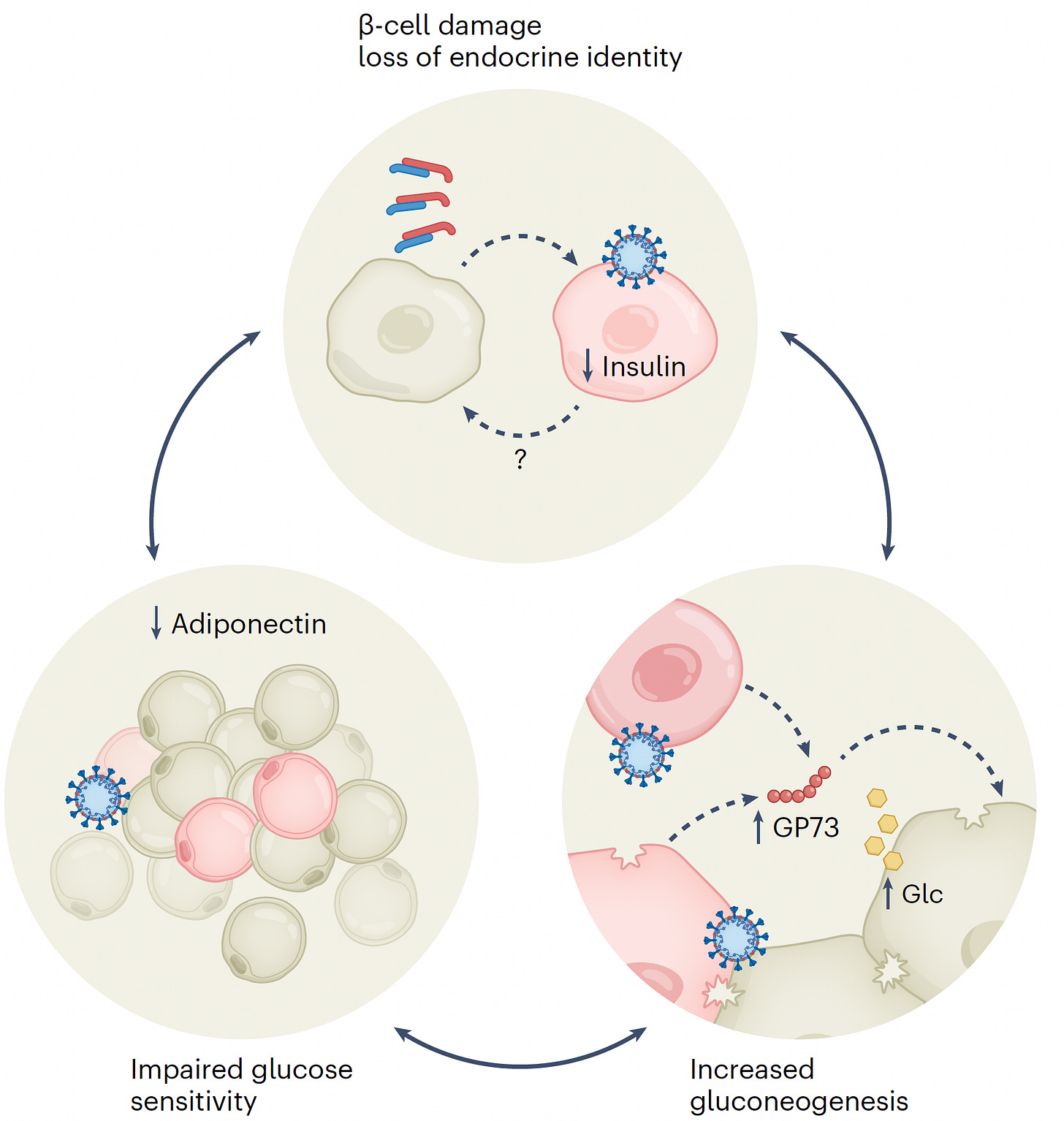
The potential role of SARS-CoV-2 infection of adipose tissue was highlighted in an experimental study (hamsters) and in the assessment of adiponectin in Covid patients
The rise of risk of Type 1 diabetes in children, the demonstration of anti-islet antibodies in some people with severe Covid, and the prospect of protection via vaccination bring to mind the contribution of auto-immunity as an additional pathway. Several viruses have been associated with risk of autoimmune diseases and for some, like hepatitis C, treatment of the virus reduces the risk of incident diabetes.
It is certainly possible and even likely that more than one of these mechanisms are operative to explain the consistent increased risk.
In our comprehensive review of Long Covid earlier this year we emphasized diabetes as one of the sequelae. It is important to note that symptoms of Long Covid and organ hits, such as diabetes (or heart, kidney, brain), are often dissociated, yet both are manifest well after the infection and can be quite durable. Another way of saying that is that people can develop diabetes after Covid without the typical symptoms of Long Covid.
In summary, Type 2 diabetes has been consistently shown to be an adverse outcome in the sub-acute (beyond 30 days) time after a Covid infection. The precise mechanism remains uncertain (along with its duration), yet there are several potential explanations, and more than one may well be contributing. If we go back to the early days of the pandemic, this was not at all anticipated. Now it’s certain and adding to the already large burden of diabetes in the population. While we know male sex, obesity, and advanced age are some risk factors, it’s not that predictable and can certainly occur in younger individuals with mild Covid. It’s all the more reason to prevent Covid infections and reinfections.






.png)

Comments
Post a Comment