Remdesivir vs Bebtelovimab vs Sotrovimab: Effective against Omicron?
In this article, we will do a roundup and cover 3 popular anti-viral treatments i.e. Remdesivir, Bebtelovimab and Sotrovimab.
Bebtelovimab (Monoclonal Antibody)
Bebtelovimab is an US FDA-authorized investigational monoclonal antibody treatment that was developed by Eli Lilly. |
| Credit: Design Cells/SPL |
When it was authorized: February 2022.
Who can get it: Adults and children ages 12 and up who weigh at least 88 pounds. They must have a positive COVID-19 test result and be at high risk for developing severe COVID-19.
How you take it: An intravenous injection is given for at least 30 seconds. Patients are observed by a health care provider for at least an hour after injection. Bebtelovimab must be given within seven days of symptom onset.
Side effects: There is limited information known about the safety and effectiveness of bebtelovimab for the treatment of mild-to-moderate COVID-19, according to the FDA fact sheet. The sheet also provides a list of potential side effects the FDA recommends reporting to a medical provider, and reports that allergic reactions can happen during and after injection. Because bebtelovimab is still being studied, it’s possible that all of the risks aren’t yet known.
How it works: It binds to the spike protein that causes COVID-19, similar to other monoclonal antibodies that have shown efficacy against hospitalization and death from the disease.
How well it works: The EUA for bebtelovimab was supported by clinical and nonclinical data that showed it has efficacy against Omicron and its BA.2 subvariant. The clinical data was based on a Phase 2 trial that treated non-hospitalized patients with bebtelovimab alone or together with another drug called etesevimab. That study is available in a preprint, which has not yet been peer-reviewed.
What else you should know: There is limited experience treating pregnant women or breastfeeding mothers. So, those patients should discuss their options and specific situation with their health care provider.
The NIH considers this to be an alternative treatment, which should be used only when neither of the NIH-preferred therapies (Paxlovid and remdesivir) are available, feasible to use, or clinically appropriate.
More information: FDA bebtelovimab fact sheet for patients, parents, and caregivers.
Remdesivir
What is it? The antiviral treatment remdesivir, sold under the brand name Veklury, was the first COVID-19 therapy to get full FDA approval, and, so far, it's still the only one. Although originally used in COVID-19 patients only after they were hospitalized, new data suggests it can be helpful in outpatients who become infected and who are at high risk for severe disease. It is meant for people who have a current COVID-19 infection.When it was authorized: Full approval was granted in October 2020. (It was first authorized in May 2020 for critically ill patients who were being treated with oxygen for COVID-19.) The authorization was later expanded to include other groups, and it was authorized to treat non-hospitalized patients in January 2022.
Who can get it: Fully approved for children and adults who are at high risk for severe disease. Infants and children must be at least 28 days old, weigh over 6.5 pounds or more, and be either hospitalized or at high risk for severe illness.
How you take it: Via injection or IV and administered only in a health care setting by a health care professional. For outpatients, the treatment is a three-day course of infusions that must be initiated within seven days of symptom onset.
Side effects: Nausea is the most common side effect. Hypersensitivity, including infusion-related and anaphylactic reactions, has been observed following treatment. There is insufficient data on the safety of using remdesivir in pregnant women or women who are breastfeeding; patients should speak with their health care provider.
How it works: Administered intravenously to patients who are in the hospital or in an ambulatory setting, the drug inserts itself into new viral genes to block replication of the virus, shortening the time it takes seriously ill patients to recover. A number of experts believe that the drug may work best early in the course of an infection.
How well it works: 87% reduction in risk of hospitalization in non-hospitalized patients given a three-day course, according to a study published in The New England Journal of Medicine in December 2021.
What else you should know: For hospitalized patients, research in early 2020 showed that the therapy reduced length of stay (the number of days in the hospital) from 15 days to 12. However, questions have been raised about remdesivir’s trial results for hospitalized patients. In late 2021, the World Health Organization (WHO) recommended against remdesivir after releasing data that showed disappointing results. Still, many U.S. hospitals continue to provide this medication.
This is one of two NIH-preferred therapies (after Paxlovid) for COVID-19.
More information: Gilead remdesivir fact sheet for patients.
Sotrovimab
The COVID-19 Treatment Guidelines Panel’s Statement on Therapies for High-Risk, Non-hospitalized Patients With Mild to Moderate COVID-19 (Last Updated: April 8, 2022)
The Panel’s recommendations take into account the efficacies of these drugs and the high prevalence of the Omicron VOC. When resources are limited, therapy should be prioritized for patients who are at the highest risk of progressing to severe COVID-19.
The Panel’s current outpatient treatment recommendations are as follows (in order of preference):
- Paxlovid (nirmatrelvir 300 mg plus ritonavir 100 mg) orally twice daily for 5 days
- Remdesivir 200 mg IV on Day 1 followed by remdesivir 100 mg IV on Days 2 and 3
- Bebtelovimab (monoclonal antibody from Eli Lilly)
- Molnupiravir 800 mg orally twice daily for 5 days
Remdesivir vs Bebtelovimab vs Sotrovimab against Omicron BA.2.12.1, BA.4, and BA.5 Subvariants | NEJM
A lab study published in July 2022 (NEJM) using the live-virus Focus Reduction Neutralization Testing (FRNT) method revealed that bebtelovimab seems to be the most promising monoclonal antibody against the BA 5 subvariant.
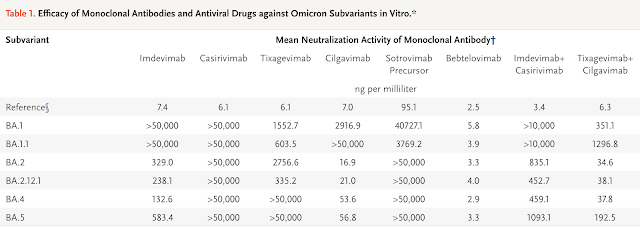
Overall, the study data also revealed that the three antiviral drugs remdesivir, molnupiravir, and nirmatrelvir (Paxlovid) may still have therapeutic value against the sublineages BA.2.12.1, BA.4, and BA.5 of SARS-CoV-2 omicron variants.

Do take note of the limitation of this study as this is a non-clinical study (not in humans). There is lack of clinical data on the efficacy of these monoclonal antibodies and antiviral drugs for the treatment of patients infected with BA.4 or BA.5 subvariants. Therefore, the selection of monoclonal antibodies or anti-virals to treat patients who are infected should be carefully considered based on the potential risks as compared to its potential benefits.


Key Takeaways
Information provided in this article is for general informational purposes only. No content in the articles should ever be used as a substitute for medical advice from your doctor or other qualified clinician. Always seek the individual advice of your trusted health care provider with any questions you have regarding a medical condition.






.png)

Comments
Post a Comment